The Egyptians could have been building structures out of concrete if they had only known the secret to making hydraulic cement. In the early 19th century, a bricklayer named Joseph Aspdin in Leeds, England first made Portland cement by burning powdered limestone and clay in his kitchen stove. He named it Portland cement because of its similarity to Portland stone, a type of building stone that was quarried on the Isle of Portland in Dorset, England. The secret to Portland cement are the compounds belite (Ca2SiO4) and alite (Ca3O·SiO4). When they are mixed with water (hydrated) they form crystals that grow like tiny rock-hard fingers wrapping around the sand and gravel creating concrete. This means that concrete will harden underwater. Concrete doesn’t harden because it dries out. It hardens because the Portland cement (which is a hydraulic cement) uses water to form crystals within the matrix of the concrete. The compounds responsible for this are created by heating a mixture of ground limestone and clay (or shale) to temperatures between 1400-1450 °C.
A modern manufacturing process consists of three stages:
1.) Grinding a mixture of limestone and clay or shale to make a fine "rawmix.
The limestone contributes calcium carbonate while the clay or shale provides the silicon and aluminum oxides needed to form belite and alite. Limestone with some impurities is preferred to limestone that is pure calcium carbonate.
2.) Heating the rawmix to a sintering temperature of (1400–1450 °C)
This fuses the rawmix into lumps or nodules which is called “clinker”. The clinker once cooled is relatively stable and can be stored.
3.) Grinding the resulting clinker to make cement
The grinding is usually done with gypsum to facilitate the grinding and to prevent flash setting (premature loss of workability or plasticity of cement paste) of the cement
Grinding the limestone to a fine enough powder before mixing it with clay would be difficult with the grinding technology of the Ancient Egypt so it would be better to comminute the limestone by burning and slaking than by grinding. This would be done by heating the limestone to 900°C for several hours which would turn the limestone into quick lime. After it has cooled, the quicklime would be combined with water to form slaked lime. This slaked lime would be combined with the clay or shale to form the raw mix and then steps 2 and 3 would be resumed.
In the second stage as the rawmix is heated, these chemical reactions take place as the temperature of the rawmix rises:
- 70 to 110 °C – Free water is evaporated.
- 400 to 600 °C – clay-like minerals are decomposed into their constituent oxides; principally SiO2 and Al2O3. Dolomite (CaMg(CO3)2) decomposes to calcium carbonate, MgO and CO2.
- 650 to 900 °C – calcium carbonate reacts with SiO2 to form belite (Ca2SiO4).
- 900 to 1050 °C – the remaining calcium carbonate decomposes to calcium oxide and CO2.
- 1300 to 1450 °C – partial (20–30%) melting takes place, and belite reacts with calcium oxide to form alite (Ca3O·SiO4).
Alite is the characteristic constituent of Portland cement. Typically, a peak temperature of 1400–1450 °C is required to complete the reaction. The partial melting causes the material to aggregate into lumps or nodules, typically of diameter 1–10 mm. This is called clinker.
The clinker once ground with gypsum is a hydraulic cement than can then be used to create concrete. Below is the depiction of a modern rotary kiln. Ground limestone and clay enter as raw materials and clinker falls out the other end.
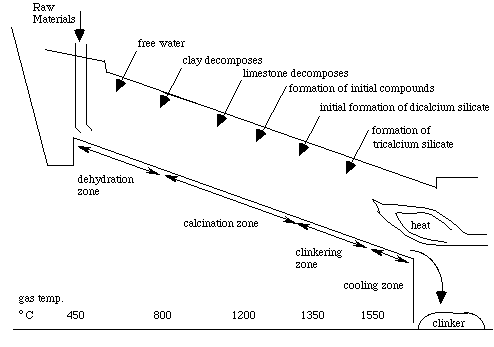
Image from http://911research.wtc7.net/cache/wtc/evidence/principles_portlandcement.html
