 |
True. Charcoal can be made from anything containing carbon. Traditionally wood has been the raw material used to make charcoal. Wood consists of three main components: cellulose, lignin and water. These compounds are composed almost entirely from atoms of hydrogen, oxygen and carbon. Charcoal is made by removing the hydrogen and oxygen in the wood while leaving just the carbon.
Making charcoal consists of 4 steps.
1.) Drying the wood to be made into charcoal.
2.) Heating the wood in an oxygen limited environment. Limiting the oxygen keeps the process from turning into full combustion which would reduce the wood to ash. As the wood heats the following changes occur:
- At 100°C the chemical bonds begin to break.
- 100° to 200°C, noncombustible products, such as carbon dioxide, traces of organic compounds and water vapor, are produced.
- Above 200°C the celluloses break down, producing tars and flammable volatiles. If these are mixed with air and heated to the ignition temperature, combustion reactions occur. That is why it is important to keep the environment oxygen poor.
- Above 200°C the lignin in the wood starts to breakdown in an exothermic reaction. This releases additional energy which can cause the temperature of the wood to rise to 400°C or more.
3.) The wood should continue to be heated to between 450- 500°C. A temperature of 500°C gives a typical fixed carbon content of about 85% and a volatile content of about 10%. The yield of charcoal at this temperature is about 33% of the weight of the oven dry wood.
4.) The wood is allowed to cool in an oxygen limited environment to prevent the oxidation (combustion) of the remaining carbon.
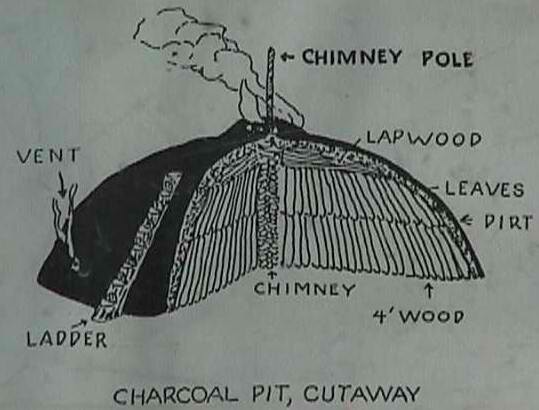 |
Traditionally this was done by piling dry wood into a dome shaped mound. The mound was then covered with smaller branches, leaves and finally dirt. Covering the mound limited its exposure to oxygen. A flue was left open in the middle of the mound to introduce hot coals and start the mound to smoldering. The shape of the mound is important because as the wood transforms to charcoal it shrinks in size. This shrinking inevitably causes holes in the outer covering of dirt which allows more oxygen into the mound. These holes have to be plugged quickly or the mound will catch fire and all the potential charcoal will go up in smoke. Planning for the shrinking of the mound helps to minimize the work in plugging holes as they appear. The key is to keep the entire mound smoldering but not burning. This requires constant attention. If there isn’t enough oxygen the mound will cool too much and if there is too much oxygen the mound will catch fire. The Swiss have been making charcoal this way since the Middle Ages. It might take two men three weeks to build the mound of 700kg (1500lbs) which will then burn for 12-18 days. The charcoal burner must spend the entire time by the mound tending to it as it smolders.
 |
A more modern method (and one that requires less attention) is to seal up the wood in a fireproof container with a small hole in it so that it can vent the gasses that are produced and then place the container in a fire or kiln. This allows more attention to be paid to the fire that is providing the heat without having to worry that the wood being charred is being exposed to too much oxygen. Here a steel barrel has been converted to a charcoal kiln. The wood would be loaded into the barrel and then it would be sealed up. A fire would be lit underneath the barrel to provide the necessary heat. Note how it vents the gasses from the barrel back into the fire with the tube that comes out of the barrel. This set up could be made much more efficient if the barrel were surrounded by earth or bricks to help trap the heat. (Photo from http://www.instructables.com/id/How-to-Make-some-Charcoal/)
Odd Notes: The process of driving off the hydrogen and oxygen by thermal decomposition is called pyrolysis. Pyrolysis is the same process that is used to turn coal into coke. It is also used to make carbon fiber. When carried to an extreme so that it leaves mostly carbon residue it is called carbonization.
References:
http://www.reuters.com/article/2008/09/03/us-swiss-charcoal-idUSLQ29563720080903
